Judi Health Policy Pulse: 2025 Regulatory Roundup, the Push for PBM Reform

2025 has been another busy year for pharmacy benefit manager (PBM) reform efforts at the federal and state levels. Five traditional PBM business practices targeted by more than 170 bills1 this year include:
- Ban on “spread pricing” – this is a core provision across federal and state bills that eliminates a PBM’s ability to earn hidden profits by charging a client more than it pays the pharmacy for the medication a plan member needs.
- Mandatory pass-through of rebates – 100% of pharmaceutical manufacturer rebates must be passed through to health plans or consumers, preventing PBMs from retaining those dollars as a source of profit.
- Delinking compensation – PBMs can only earn a fee based on the administrative services they provide, not the fulfillment or dispensing of medication to plan members.
- Transparency and reporting requirements – PBMs must disclose pricing, rebates, fees, and other data to regulators, health plans, and plan sponsors.
- Anti-steering and network neutrality – PBMs cannot force plan members to use affiliated pharmacies or discriminate against independent pharmacies.
States Have Led the Way on PBM Reform, but…
The push for PBM transparency at the federal level has accelerated recently.
Signed into law in July, the One Big Beautiful Bill Act (OBBBA; H.R. 1 of the 119th Congress) included several provisions impacting PBMs and pharmacies that provide solutions and services to state Medicaid plans and their members. The most notable provisions expand the orphan drug exemption and delay Medicare drug price negotiations. Under OBBBA, drugs with multiple rare disease indications remain exempt, and negotiations begin only after a drug receives a non-orphan approval—allowing manufacturers to maintain higher prices up to that point. Additionally, OBBBA narrows Medicaid eligibility, creating a more administratively demanding environment for states.
More recently, the Lower Health Care Premiums for All Americans Act, which the U.S. House of Representatives passed on December 17, 2025, expands association health plans for small businesses and self-employed individuals, clarifies rules for stop-loss insurance, and introduces CHOICE accounts to let employees use pre-tax dollars for individual coverage. The bill proposes significant PBM-related transparency requirements – mostly related to drug-level reporting – and restores cost-sharing reduction payments for ACA silver-tier plans starting in 2027. However, it does not address ACA premium tax credits and faces an uphill battle in the Senate.
The Pharmacy Benefit Manager (PBM) Price Transparency and Accountability Act, introduced in early December by U.S. Senate Finance Committee Chairman Mike Crapo, R-Idaho., and Ranking Member Ron Wyden, D-Oregon, delinks PBM compensation from negotiated rebates, prohibits PBM spread pricing for Medicaid, increases PBM reporting requirements to Medicare Part D plan sponsors, and reinforces existing requirements that plan sponsors contract with any willing pharmacy. This is essentially the same PBM reform legislation that was introduced at the end of 2024, and we are optimistic that it will be considered in the new year.
Additionally, HR 4317, the PBM Reform Act of 2025, was introduced earlier in the year. This bill would ban spread pricing in Medicaid, require PBMs to pass 100% of manufacturer rebates to plan sponsors, and delink PBM compensation from drug prices in Medicare Part D. It also would require detailed reporting and would grant federal agencies broad authority to audit and monitor PBM practices.
And then there's TrumpRx and Delivering Most-Favored-Nation Prescription Drug Pricing to American Patients:
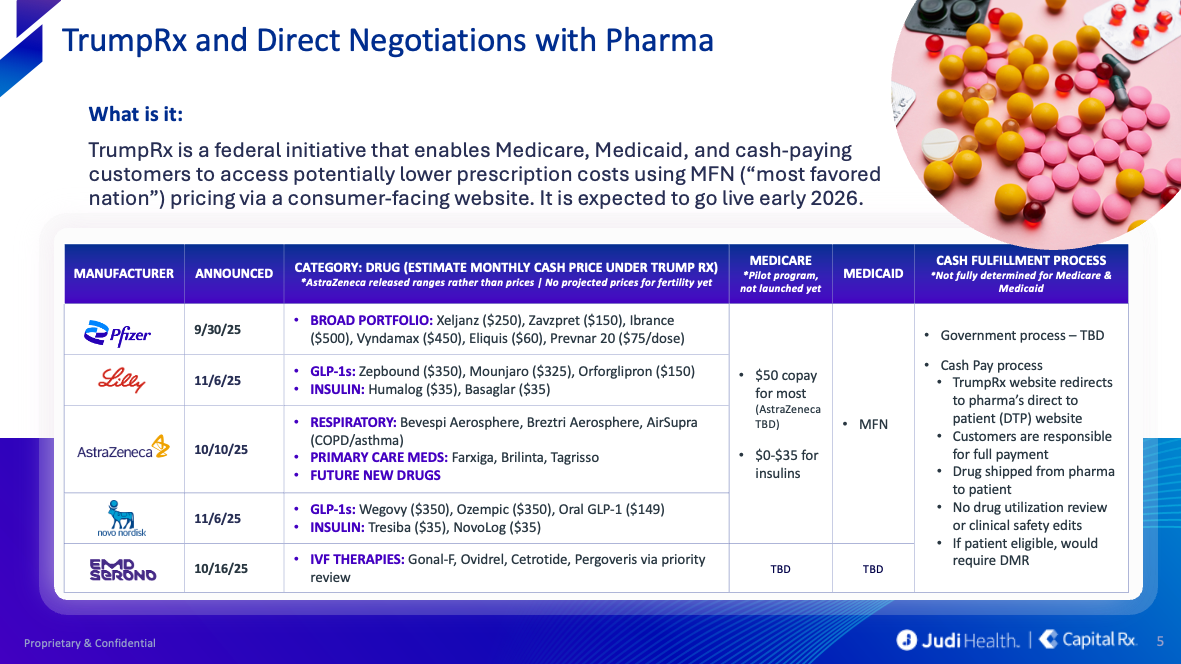
PBM Reform at the State Level
Arkansas Act 624
Signed into law by Governor Sarah Sanders on April 16, 2025, Arkansas Act 624 established a first-of-its-kind statute that, effective January 1, 2026, would have prohibited PBMs from owning or operating pharmacies within the state. The law directed the Arkansas State Board of Pharmacy to revoke or not renew pharmacy permits for any pharmacies owned by a PBM or its affiliates, with limited exceptions for employee-only pharmacies and allowing temporary permits for rare or specialty drugs. As the law applies to out-of-state PBMs operating mail order pharmacy services, the statute could disrupt prescription access for tens of thousands of Arkansas patients.

On July 28, 2025, a U.S. District Court Judge for the Eastern District of Arkansas granted a preliminary injunction blocking enforcement of the law, finding that it likely violates the U.S. Constitution’s Commerce Clause by discriminating against out-of-state companies and is preempted by TRICARE, the federal military health benefits program. The Arkansas State Board of Pharmacy filed a notice of appeal on July 31, 2025. While the appeal pends, the preliminary injunction preserves the status quo and allows PBM-affiliated pharmacies to continue operating in Arkansas pending final resolution.
California SB 41 and SB 306
California has enacted sweeping reforms that fundamentally change how PBMs operate in the state. Effective January 1, 2026, Senate Bill (“SB”) 41 prohibits PBMs from requiring the use of only affiliated pharmacies, prohibits discrimination against non-affiliated pharmacies, limits PBM income to PBM fees, and requires the use of a pass-through pricing model. This represents one of the most significant regulatory changes to PBM operations in California's history, and it mandates the exact operational model championed by Capital Rx: pass-through pricing, 100% rebate pass-through, prohibition of spread pricing, and restricting PBM income to management fees only.
Another recently enacted bill, SB 306, allows the Department of Managed Health Care and the Department of Insurance to waive prior authorization requirements for healthcare services or prescriptions that health plans and insurers approve at least 90% of the time. The law requires all insurers to report detailed data on prior authorization approvals, modifications and denials by Dec. 31, 2026, which will help identify services frequently approved. By July 1, 2027, the departments must publish a list of those services, and by no later than January 1, 2028, plans and insurers must stop requiring prior authorization for them.
Colorado HB 25-1094
In May 2025, Colorado enacted House Bill (“HB”) 25-1094, fundamentally restructuring PBM operations effective January 1, 2027. The law applies to PBMs serving Fully Insured, Exchange, and Medicaid health plans. The law requires PBMs to obtain Colorado Division of Insurance licenses and completely overhauls their compensation structure by prohibiting spread pricing, rebate retention, and any percentage-based arrangements. Instead, PBMs may only collect transparent flat-dollar service fees per prescription, with all other income credited back to health plans.
The law establishes strict pharmacy reimbursement standards, requiring PBMs to pay pharmacies at or above the National Average Drug Acquisition Cost (NADAC) plus a reasonable dispensing fee. Formulary design is also regulated — PBMs cannot favor branded drugs over generics unless cost-based exceptions apply. Additionally, restrictive contract terms are prohibited, meaning PBMs cannot include gag clauses that prevent pharmacies or health plans from disclosing cost information or discussing lower-cost alternatives with patients.
Transparency requirements are extensive. PBMs must disclose cost and income data to health plans, permit audits, and submit annual reports on rebates, fees, and cost data to the Division of Insurance. The Division gains broad enforcement authority, including rule-making power and the ability to impose fines and license actions under Colorado insurance law.
Illinois HB 1697
On July 1, 2025, Governor J.B. Pritzker signed HB 1697, the Illinois Prescription Drug Affordability Act, enacting sweeping reforms to PBM practices. The law applies to PBMs serving Fully Insured, Exchange, Self-Funded ERISA, Medicare, Medicaid, and other regulated government health plans. The law bans spread pricing and prohibits PBMs from steering patients to affiliated pharmacies, while requiring 100% pass-through of manufacturer rebates to health plans.
New transparency mandates include annual reporting of drug costs, rebates, and fees to the Department of Insurance, with the Department authorized to conduct annual audits of PBM records. To support independent and rural pharmacies, the Act imposes a $15 per-covered-individual annual assessment on PBMs to fund targeted grants for these providers.
Consumer protections limit point-of-sale costs to the lowest of three amounts: the plan's cost-sharing requirement, the retail price without coverage, or any available discounted price through manufacturer programs or vouchers. The law has a split effective date — PBM registration, assessments, and reporting requirements took effect immediately upon signing, while the substantive reforms including spread pricing bans, steering prohibitions, rebate pass-through requirements, and consumer cost-sharing protections apply to health plans amended, delivered, issued, or renewed on or after January 1, 2026.
Iowa SB 383
On July 1, 2025, Iowa imposed significant new requirements on PBMs operating in the state via SB 383. The law applies to PBMs serving Fully Insured, Exchange, Self-Funded ERISA, Medicare, Medicaid, and other regulated government health plans. The law mandates 100% pass-through of manufacturer rebates to health plans and bans spread pricing entirely. PBMs must reimburse independent retail pharmacies at the NADAC plus a $10.68 dispensing fee.
The law also prohibits patient steering to preferred pharmacies and bars PBMs from discriminating among pharmacies for network participation or reimbursement purposes. All requirements took effect immediately upon the law's passage.
On July 21, 2025, the U.S. District Court for the Southern District of Iowa issued a preliminary injunction requested by a coalition of Iowa employers and ERISA-governed health plans who had challenged the law. This preliminary injunction blocks enforcement of several key provisions, including the mandated $10.68 dispensing fee, anti-steering and anti-discrimination rules, and requirements that all patient payments count toward deductibles. The court found these provisions likely preempted by ERISA and, in some cases, violative of the First Amendment, but allowed other transparency and reporting requirements to stand.
The injunction is limited to the named plaintiffs and their PBMs, so the law remains in effect for other entities while the litigation continues.
Louisiana Act 474
On June 20, 2025, Governor Jeff Landry signed Louisiana HB 264 (Act 474) into law, establishing extensive PBM oversight requirements emphasizing transparency and fair reimbursement. The law requires PBMs to pass all manufacturer rebates through to plan sponsors and apply them to reduce premiums, cost-sharing, or expand coverage. PBMs must disclose all administrative and management fees in client contracts and are prohibited from using spread pricing or effective rate pricing for local pharmacies.
The legislation mandates an appeals process allowing pharmacies to challenge claim payment errors and receive retroactive adjustments when errors are confirmed. PBMs must certify annual compliance and submit detailed transparency reports to the Louisiana Department of Insurance. The law creates the Pharmacy Benefit Manager Enforcement Fund and grants the Commissioner authority to audit PBMs, review compensation programs, and impose penalties for violations.
The law has staggered effective dates—rebate pass-through and reporting requirements took effect immediately upon enactment, while local pharmacy reimbursement rules and the appeals process become effective January 1, 2026.
Texas SB 1236
In June 2025, Texas passed legislation that strengthens state oversight of PBMs and health benefit plan issuers by restricting retroactive claim adjustments after adjudication. Post-adjudication changes are limited to cases involving fraud, duplicate payments, or substantive dispensing errors. Recoupment amounts are capped—drug cost plus dispensing fee for serious errors, and dispensing fee only for clerical errors.
Effective: September 2025 (for new and renewing plans) and January 2026 (all existing plans), Texas SB 1236 also prohibits participation fees—defined as any upfront charges such as application, credentialing, or network access fees that a pharmacy might be required to pay to join or remain in a PBM network—before a pharmacy is allowed to review the full proposed contract. It requires PBMs and health benefit plan issuers to provide pharmacies with secure online access to all contracts and addendums, including advance notice before material changes. The regulation further grants the Texas Department of Insurance expanded enforcement authority, including audit powers and substantial penalties for violations.
Medicare 2026 Overview for Plan Sponsors
2026 Final Rule
CMS has finalized comprehensive regulatory updates for Medicare Advantage and Part D plans for 2026, codifying Inflation Reduction Act provisions and establishing new operational standards that will significantly impact plan administration. The final rule eliminates deductibles for vaccines, caps insulin costs at $35 or 25% of the negotiated price (whichever is lower), and introduces a monthly payment option for participants in the Medicare Prescription Payment Plan with automatic renewal features.
For reference and additional information, including links to other CMS resources, please visit: Final CY 2026 Part D Redesign Program Instructions
Additionally, CMS has codified the 30-day window for timely Prescription Drug Event (PDE) submissions and implemented Maximum Fair Price requirements on select drugs, creating a more structured framework for plan sponsors to navigate the evolving Medicare Part D landscape while ensuring enhanced affordability protections for beneficiaries.
CMS finalized broad regulatory updates for Medicare Advantage and Part D, codifying
IRA provisions and introducing new standards for plan operations.
2026 Medicare Part D Redesign (MPF)
The Inflation Reduction Act continues to fundamentally reshape Medicare Part D benefit design for 2026, with CMS finalizing significant updates that include the implementation of negotiated drug prices and a new subsidy program that will alter cost-sharing dynamics across the program. The standard benefit design features an increased out-of-pocket cap of $2,100 (up from $2,000 in 2025) and a deductible of $615, while CMS will provide a 10% subsidy on negotiated prices for selected drugs and implement a revised liability-sharing structure among sponsors, manufacturers, and CMS across different benefit phases.
These changes will deliver greater affordability and cost predictability for beneficiaries while requiring plan sponsors to substantially adjust their plan designs and actuarial models to accommodate the new cost-sharing framework, creating both opportunities for enhanced member value and operational challenges in benefit administration.
2026 Pricing Files Test Submissions
CMS has released comprehensive guidance for 2026 Part D pricing data submissions, establishing a structured timeline and enhanced validation requirements that emphasize accuracy and compliance with the Drug Price Negotiation Program. The submission process includes three test periods between June and August 2025 with final test submissions due August 20, followed by production submissions due September 8-11, 2025, and public display beginning October 1, 2025.
New validation requirements include checks for maximum fair prices and custom dispensing fees, while sponsors now have the option to automate submissions through the HPMS API for improved efficiency. These enhanced validation protocols and compressed timelines require sponsors to prioritize data integrity and implement robust quality assurance processes to ensure timely compliance, as the increased scrutiny of pricing data reflects CMS's commitment to transparency and accuracy in the evolving Medicare Part D landscape.
The CMS has also released detailed guidance for 2026 Part D pricing data submissions, emphasizing accuracy and compliance with the Drug Price Negotiation Program.
2026 Maximum Fair Price (MFP)
Under the Inflation Reduction Act, CMS gained unprecedented authority to negotiate drug prices for high-cost, single-source drugs under Medicare Part D, with the first cycle of negotiations concluding in 2024 and Maximum Fair Prices (MFPs) for 10 selected drugs taking effect January 1, 2026. The MFP framework applies to drugs without generic or biosimilar competition, with CMS considering research and development costs, therapeutic alternatives, and patient input during the negotiation process, while the resulting prices exclude dispensing fees and may vary by National Drug Code (NDC).
For insulin products subject to MFP, patient costs will be capped at the lower of $35 or 25% of the Maximum Fair Price, creating additional affordability protections for diabetic beneficiaries. This landmark pricing intervention is expected to significantly reduce Medicare spending and lower out-of-pocket costs for affected medications, requiring plan sponsors to proactively adjust their formulary strategies and benefit designs to accommodate these negotiated prices while maintaining competitive positioning in the Medicare Advantage and Part D marketplace.
Mental Health Parity Enforcement Paused: Key 2025 Developments
2025 has also brought major shifts in Mental Health Parity enforcement, with new federal litigation and a significant enforcement pause under the Trump administration affecting the Mental Health Parity and Addiction Equity Act (MHPAEA) and Consolidated Appropriations Act of 2021 (CAA). Existing MHPAEA statutory requirements and the 2013 Final Rule remain in effect.
Why enforcement is paused:
- Legal Challenge: In January 2025, the ERISA Industry Committee (ERIC) sued to invalidate the 2024 Final Rule, arguing it exceeded statutory authority and imposed unworkable compliance burdens.
- Policy Shift: The Trump administration directed agencies to review regulations that lack statutory support or impose undue burdens. In May 2025, the Departments requested a litigation stay and announced a non-enforcement policy while reconsidering the rule, including potential rescission or modification.
New Requirements (that were paused) include:
- “Meaningful benefits” requirement across all classifications.
- Expanded documentation standards for Nonquantitative Treatment Limitations (NQTLs).
- Fiduciary certification of comparative analysis.
- Updated definitions for MH/SUD conditions.
- Stricter comparative analysis standards under CAA 2021.
Key enforcement relief:
- No enforcement of the new provisions introduced by the 2024 Final Rule
- 18-month grace period after any final court decision from the ERIC litigation that is challenging the rule
- 2013 Final Rule remains in effect for existing obligations
- Underlying MHPAEA statutory requirements continue to apply as amended by the CAA
Immediate Impact for Plans:
1. Outcomes-Based Compliance Standard Suspended: The "relevant data" requirement is on hold. Plans no longer need to demonstrate compliance through outcomes data. Instead, compliance reverts to the 2013 standard, where outcomes are not determinative—process and design requirements control.
2. "Meaningful Benefit" Requirement Suspended: Plans are no longer required to provide "meaningful benefits" across all benefit classifications. Under the suspended 2024 rule, if a plan covered any mental health or substance use disorder treatment in one classification, it had to offer meaningful benefits for that condition in every classification where medical/surgical benefits were available. This cross-classification requirement is now paused.
3. Fiduciary Certification Requirement Suspended: Fiduciaries are no longer required to certify their MHPAEA compliance review and oversight, including regarding non-quantitative treatment limitations (NQTLs). This administrative burden is temporarily lifted.
The pause significantly reduces administrative complexity but does not eliminate underlying parity obligations under MHPAEA and the CAA.
We look forward to providing updates on PBM reform efforts and new health policy issues via Judi Health Policy Pulse. If you enjoy this sort of content, sign up for Judi Health Insights so you never miss an update – it's one email a month, we promise!
For more information about Judi Health and Capital Rx – transparent health and pharmacy benefit administration – get in touch with our team!
References
1 2025 State Legislation to Lower Prescription Drug Costs. National Academy for State Health Policy. https://nashp.org/state-tracker/2025-state-legislation-to-lower-prescription-drug-costs/


.jpg)







